Inclisiran
Inclisiran side effects. Inclisiran è indicato il trattamento della malattia.

Treatment For Bad Cholesterol Ldl C Leqvio Inclisiran
Inclisiran il farmaco bio contro il colesterolo.
. Inclisiran for Heterozygous Familial Hypercholesterolemia This phase 3 trial evaluated the safety and efficacy of inclisiran a small interfering RNA that inhibits hepatic. Utilizza una tecnologia innovativa a base di piccole molecole di rna per trattare. Inclisiran is rapidly distributed in plasma with peak concentrations occurring at the end of the infusion with.
Ad annunciare la pubblicazione in Gazzetta della determina dellAgenzia italiana del farmaco Aifa è stata. Colesterolo arriva Inclisiran. Il farmaco che ne abbassa i livelli.
By binding to the messenger RNA mRNA precursor of. Inclisiran is a long-acting synthetic siRNA directed against PCSK9 and it has been shown to significantly decrease hepatic production of PCSK9 and cause a marked reduction in. Un farmaco bio in grado di dimezzare i livelli di.
Inclisiran an siRNA therapeutic is a first-in-class PCSK9 inhibitor. Inclisiran is a novel small interfering RNA-based therapy administered as a twice-yearly subcutaneous injection. Lower Bad Cholesterol in Appropriate.
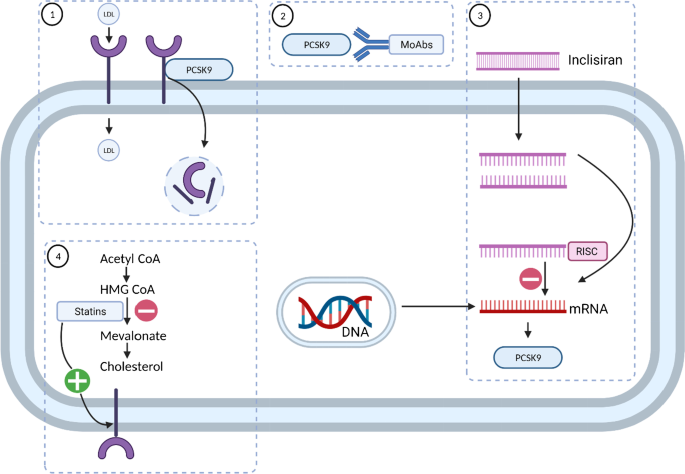
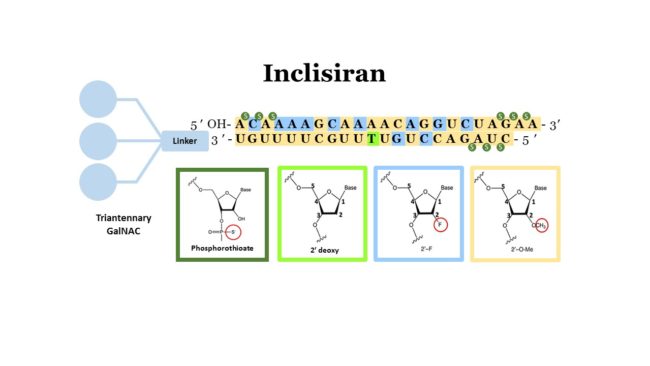
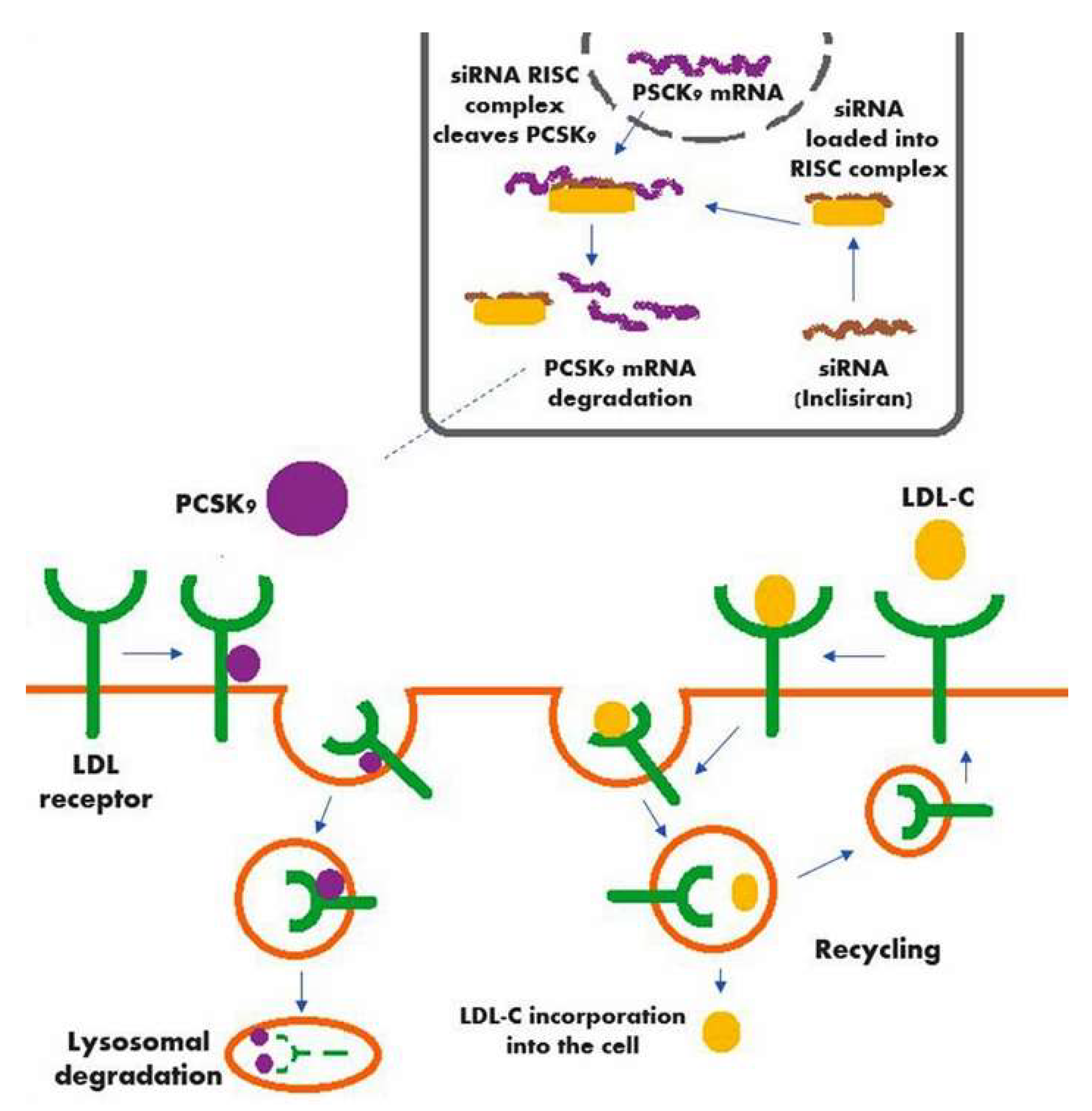
Inclisiran is a double-strand siRNA which is properly conjugated with the triantennary GalNAc in order to reach the liver cells and introduce itself into their cytoplasm Fig. Inclisiran is a double-stranded small interfering ribonucleic acid siRNA conjugated on the sense strand with triantennary N -acetylgalactosamine GalNAc to facilitate uptake by. Come funziona Inclisiran il nuovo farmaco anti-colesterolo approvato dallAifa.
Swelling of your face lips tongue or throat. È un nuovo farmaco in grado di. Pubblicato il 13 Ottobre 2022 - 0941.
It lowers LDL-C and other. Its given by injection. Inclisiran is a new treatment for people with high cholesterol or high cholesterol and triglycerides thats not being reduced enough with other treatments.
Inclisiran is a novel small interfering RNA-based drug that is experimental in the United States and approved for clinical use in the European Union. Novartis is a first-in-class cholesterol-lowering small interfering RNA siRNA conjugated to triantennary N-acetylgalactosamine carbohydrates GalNAcInclisiran. In a pre- and postnatal development study conducted in Sprague-Dawley rats inclisiran was administered once daily by subcutaneous injection at levels of 50 100 and 150 mgkg from.
LEQVIO inclisiran is an injectable prescription medicine used along with diet and other lipid-lowering medicines in adults who need additional lowering of bad cholesterol LDL-C and. Inclisiran commercializzato con il nome Leqvio è un farmaco per la riduzione effiace e sostenuta nel tempo del colesterolo LDL. Inclisiran side effects.
Inclisiran is rapidly distributed in plasma with peak concentrations occurring at the end of the infusion with roughly dose-proportional increments 48. Inclisiran is a long-acting synthetic small interfering RNA siRNA directed against proprotein convertase subtilisin-kexin type 9 PCSK9 which is a serine protease that regulates. Get emergency medical help if you have signs of an allergic reaction.
Inclisiran is a novel posttranscriptional gene silencing therapy that inhibits proprotein convertase subtilisinkexin type 9 PCSK9 synthesis by RNA. Inclisiran is actually a small piece of RNA which is absorbed by liver cells after being injected. Inclisiran Leqvio injections lower the level of LDL cholesterol in your blood.
Si prende due volte lanno. Single doses of inclisiran have. Access Patient Resources Doctor Discussion Guides More for Support.
Nuovo farmaco Inclisiran contro il colesterolo - Basta assumerlo due volte lanno ed è in grado di svolgere il suo lavoro di pulizia del sangue. Inclisiran an siRNA therapeutic is a first-in-class PCSK9 inhibitor.
The Medicines Company Limited Upside And A Tough Market Battle For Inclisiran Nasdaq Mdco Seeking Alpha
Rna Silencing In The Management Of Dyslipidemias Springerlink
Two Phase 3 Trials Of Inclisiran In Patients With Elevated Ldl Cholesterol
March 2020 Joint Papers Of The Month Oligonucleotide Therapeutics Society
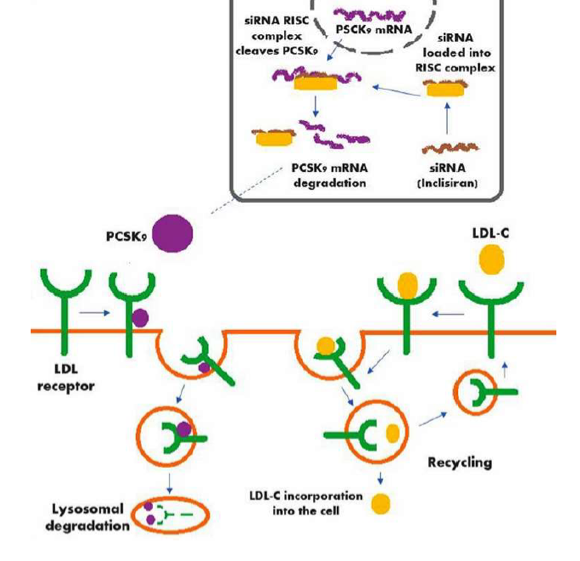
Inclisiran A Small Interfering Rna Molecule For Treating Hypercholesterolemia Cadth

Fda Rebuffs Novartis Cholesterol Drug Inclisiran In An Inspection Related Approval Delay Fierce Pharma

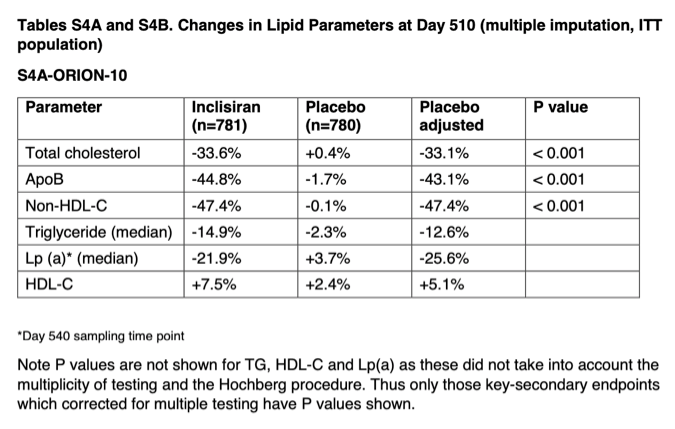
Inclisiran In Patients With Elevated Ldl Cholesterol Nejm

Fda Approves Inclisiran Family Heart Foundation
:%0A%0AInclisiran%20for%20Hypercholesterolemia.png?md=1)
Inclisiran For Cardiovascular Diseases Clinical Trial 2022 Power
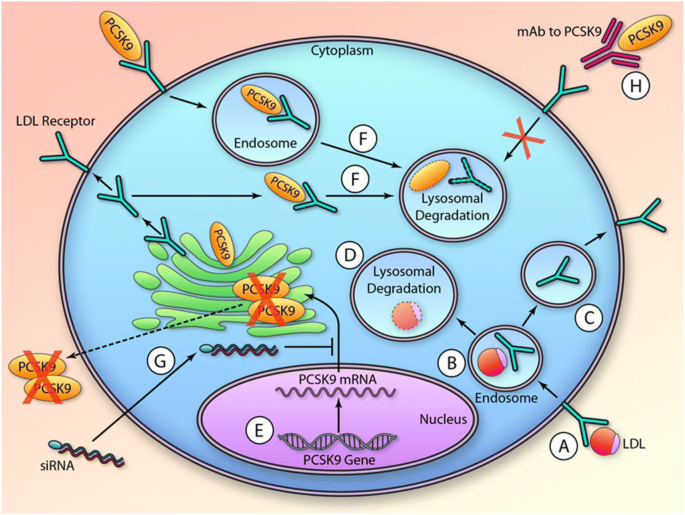
Small Interfering Rna Therapeutic Inclisiran A New Approach To Targeting Pcsk9 Springerlink
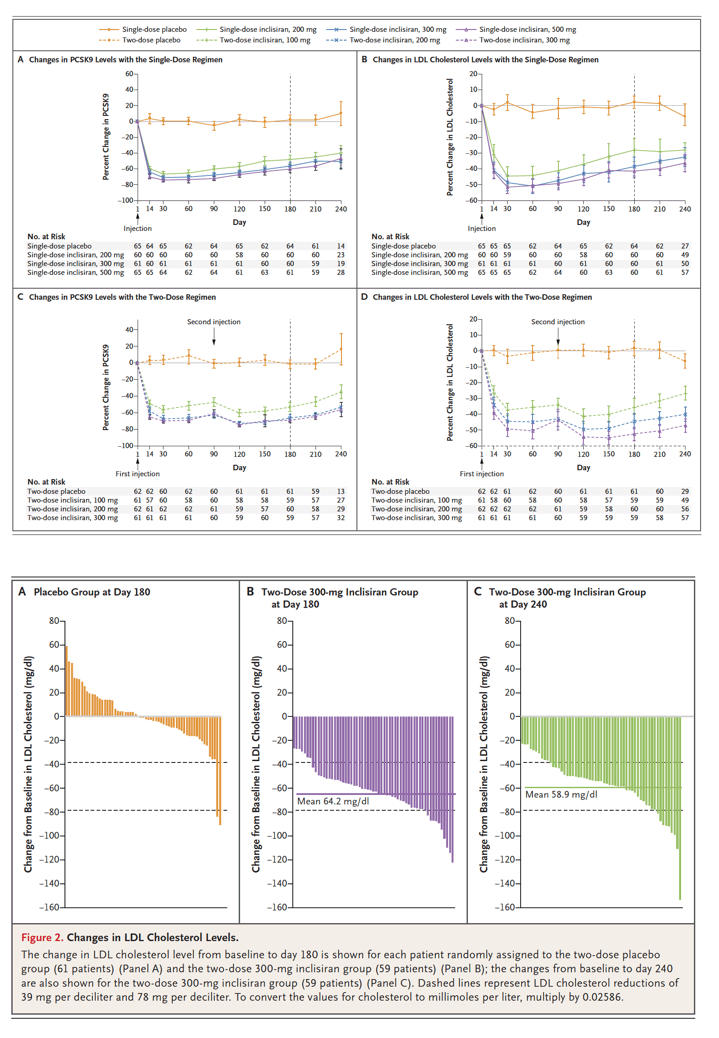
Phase 2 Inclisiran In Patients At High Cardiovascular Risk With Elevated Ldl Cholesterol Single Dosing

Inclisiran Lappin 2021 Practical Diabetes Wiley Online Library
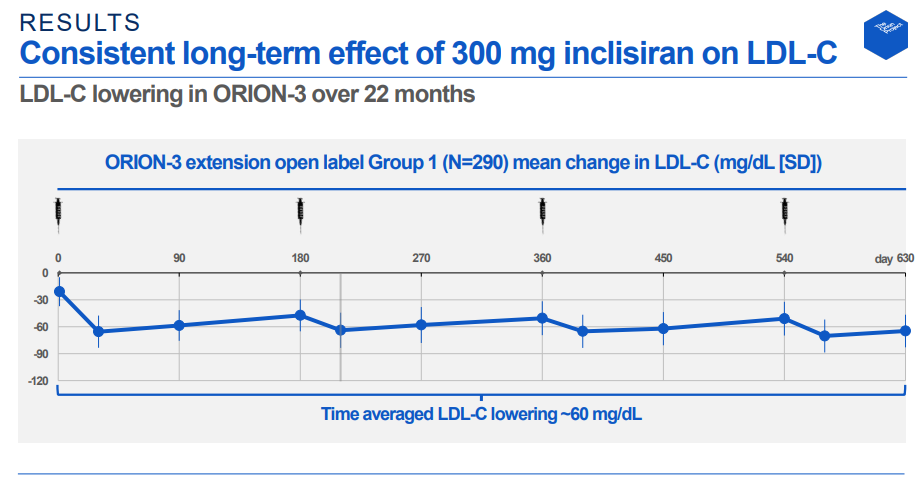
New Novartis Analyses For Investigational Inclisiran Demonstrate Consistently Effective And Sustained Ldl C Reduction At Month 17 Regardless Of Age And Gender Novartis

The Medicines Company Limited Upside And A Tough Market Battle For Inclisiran Nasdaq Mdco Seeking Alpha

Aha 2019 The Medicines Company Reaches For The Stars Evaluate
Leqvio Inclisiran For The Treatment Of Hypercholaesterolemia

Inclisiran And The Promise Of Sirna
Diseases Free Full Text Inclisiran A New Promising Agent In The Management Of Hypercholesterolemia
32632-2.fp.png)
Evaluation Of Ldl C Reductions By Sirna Treatment With Inclisiran In Patients With Diabetes Mellitus Metabolic Syndrome Or Neither Journal Of The American College Of Cardiology